Team:SUSTC-Shenzhen/Notebook/HeLaCell/Stably transfect cell with target-sequence EGFP
From 2014.igem.org
Notebook
Elements of the endeavor.
Contents |
Construct cell-line with EGFP gene which insert target sequence.
2014/10/3 Simulate cutting HIV & HBV integrate sequence by Cas9Introduction:
Our final goal is to cut integrated Lentiviruses
Oct. 3rd
Seed the plate
[2014 Oct. 3rd]
Materials
- G5 cells(HeLa cells transfected with Cas9 gene under control of Tet-On).
- DMEM with 10% FBS, Penicillin-Streptomycin and 4.4μg/mL blasticindin and 2.0 μg/mL puromycin.
Procedures
- Rinse the plate twice with PBS to remove any floating cells and wash out all medium.
- Trypsinize all of the cells and suspend with complete medium with 4.4μg/mL blasticindin and 2.0 μg/mL puromycin.
- Count cell concentration and dillute to 50,000 cells/ml. Add suspention 1ml with 50,000 cells per well 12 wells into 24-well plate.
- Incubate the 24-well plate for 12 hours.
Result
Cells are seeded very even in the well. Perfect for transfection.
Oct 5th
Transfection
[2014 Oct. 5th 11:00]
We have to stably transfect HeLa cell to construct a new cell line, so we use piggybac to integrate gene into genome.
For 3 different modified EGFP gene, we have 3 groups 4 wells per group.
Materials
- Lipofectamine® 3000 Reagent
- Opti-MEM
- DMEM with 10% FBS.
- Endo-free extract piggybac
| Target sequence | Concentration |
|---|---|
| HIV1 | 6638 ng/μl |
| HBV1 + HBV2 | 6076 ng/μl |
| HBV2 | 6653 ng/μl |
Procedures
- Dilute 0.75μl/well 9μl at total Lipofectamine 3000 reagent in 25μl/well 300μl in total Opti-MEM, and incubation for 5min
- Prepare master mix of 3 groups, DNA 0.5μg/well by dilutiong 3 plasmidsd in Opti-MEM medium 25μl/well according to the form, then add P3000 reagent 1μl/well.
- Add dilutetd DNA to each tube of diluted lipo 3000.
- Incubation for 10min
- Add DNA lipid complex to cell waiting for the harvest.
Laboratory note
| target+EGFP | Piggybac | |
|---|---|---|
| HIV1 | 0.15 | 0.345μl |
| HBV12 | 0.165 | 0.345μl |
| HBV2 | 0.15 | 0.345μl |
- Add DNA to 100μL/group Opti-MEM medium according to the table and add 4μL P3000 reagent, mix well.
- Dilute Lipofectamine 3000 Reagent in Opti-MEM Medium: 300μL Opti-MEM Medium + 9μL Lipofectamine 3000 Reagent, mix well.
- Add 100μL Diluted Lipofectamine 3000 to Diluted DNA of each group (1:1 ratio), mix well.
- Incubate for about 10min.
- Add DNA-lipid complex (25μL per well) to cells, shake the plate. (11:40 am)
- After 12h, change the medium with the complete medium with 10% FBS and Penicillin-Streptomycin.
12 hours after transfection
Passage cells
Passage cells 3 wells to one petri dish, and the other 1 well to another petri dish for monoclones.
- Wash cells with PBS, trypsin digestion, add medium to stop digestion
- Transfer cell suspension of 3 wells into a petri dish, and 1 well into another petri dish.
- Count the cell concentration with blood counting chamber.
- Mark the new dishes, add 6 mL medium to every dish, mix well.
- Culture.
Oct 6th
Add antibiotics
60 hours after transfection Add antibiotics to select cells stably tranfected.
- Blasticidin 4.4μg/ml + Puromycin 2.0μg/ml 6ml per petri dish
For killing cells have not been stably transfected.
Microscope Observation

Figure 1. Picture of cells transfected with target sequence + EGFP day one.

Figure 2. Picture of cells transfected with target sequence + EGFP day one.

Figure 3. Picture of cells transfected with target sequence + EGFP day one.
Result
From the observation, it rise a weired problem, that NLS of GFP does not work, after inserting target sequence behined gene of GFP. But the sequecing result shows that there is no shift mutation
Oct. 11th
Seed plate
Count the number of cells of each group, consider the cells are keeping round up, seed plate 200,000 cells/well. In complete medium with 1μg/ml Doxycycline.
Each group seed 2 wells.
Oct. 12th
Transfection plasmid encoding gRNA
Cleavage of DNA by Cas9 protein have to be medirate by gRNA. So we transfect plasmid encoding gRNA into cell, induce Cas9 to cut target sequence.
Materials
- 24 well plate seeded with 3 gourps of cells 2 wells per group, one as test, one as control, 6 wells at total.
- Lipofectamine® 3000 Reagent
- Opti-MEM (gibco Invitrogen®)
- 3 Endotoxin-free extraced UAS-mCherry-gRNA with gRNA targeting viral sequences and 7 UAS.
- DMEM (CORNING) (Hi-clone Thermo) with FBS(gibco Invitrogen) 1µg/ml doxycycline
- DMEM with FBS 1µg/ml and Pen-Strep
Procedures
- Discard the old medium and add DMEM with 10% FBS and 1000ng/mL doxycycline.
- Transfect cells with the plasmid 7 UAS encoding gRNA and mCherry using Lipofectamine® 3000 Reagent.
- Passage cells on the next day, culture cells in DMEM with 10% FBS Penicillin-Streptomycin and 1000ng/mL doxycycline.
- Observe the cells under a fluorescence microscope and read cells with flow cytometry in the following days.
Oct. 14th
Flow Cytometry
We use flow cytometry to read the result Cas9 cutting viral target sequence after of uas-mCherry-gRNA. gRNA
Procedures
- Remove and discard the growth medium. Rinse the plate twice with PBS to remove any floating cells and wash out all medium.
- Add about 1mL of the 0.25% trypsin to 10cm petri dish and aspirate after 30s.
- Trypsinize 1-2min at 37°C
- Add 4ml complete medium per petri dish to neutrualize trypsin stop trypsinize.
- Gently suspend with Pasteur tube or pepitte.
- Pepite 500μL into EP tubes.
- Centrifuge for 5min at 800rpm.
- Aspirate supernatant and add 500μL PBS to suspend pellet filt with 300mesh filter.and pepite to Flow cytometry tubes. Mark well and carefully.
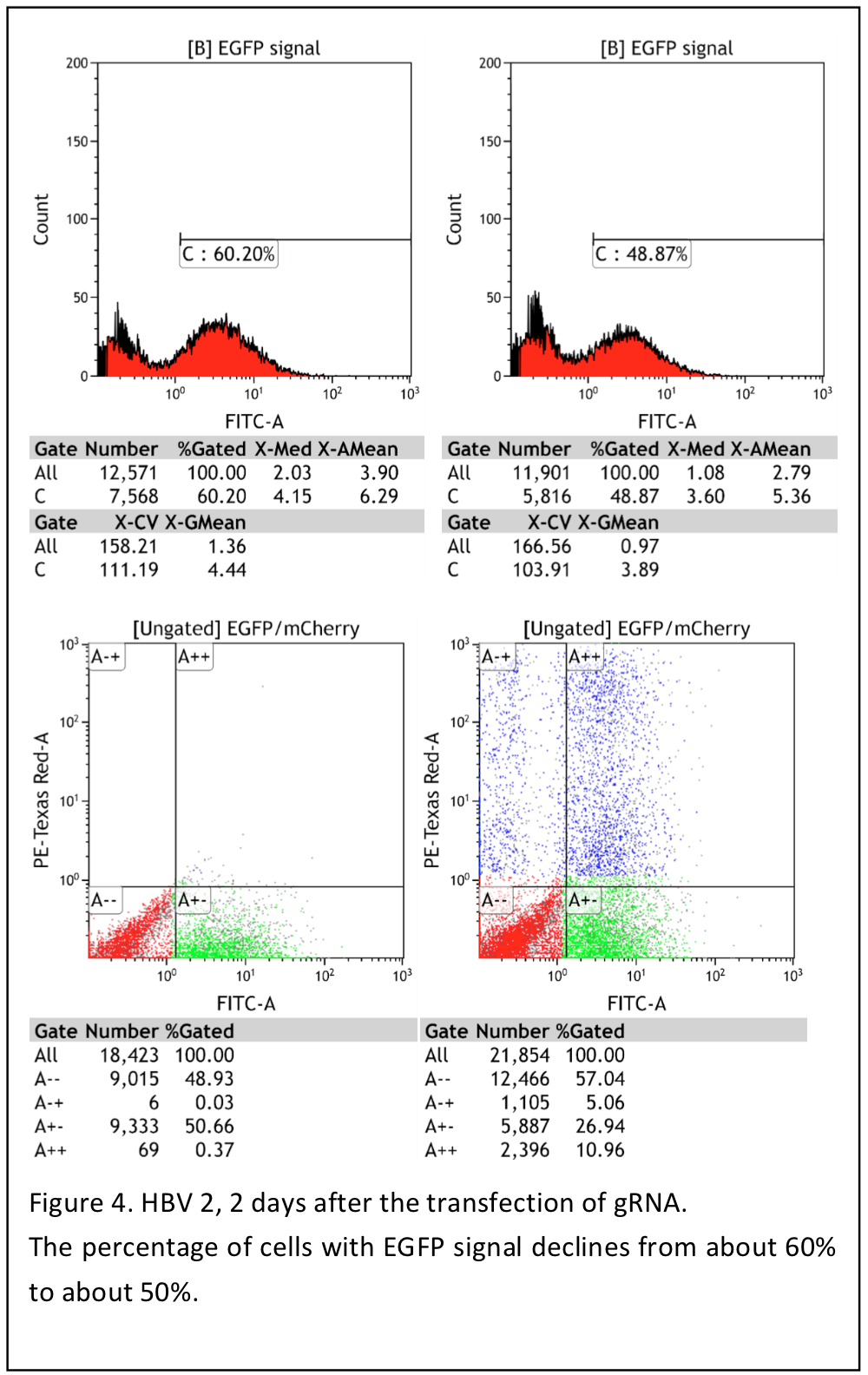
Oct. 16th
Flow Cytometry
We use flow cytometry to test the fraction of cell express fluorescence protein.
Procedure
- Remove and discard the growth medium. Rinse the plate twice with PBS to remove any floating cells and wash out all medium.
- Add about 100μL of the 0.25% trypsin to each well.
- Trypsinize 1-2min at 37°C
- Add 400μl complete medium per well to neutrualize trypsin stop trypsinize.
- Gently suspend with pepitte.
- Pepite 500μL into EP tubes.
- Centrifuge for 5min at 800rpm.
- Aspirate supernatant and add 500μL PBS to suspend pellet filt with 300mesh filter.and pepite to Flow cytometry tubes. Mark well and carefully.
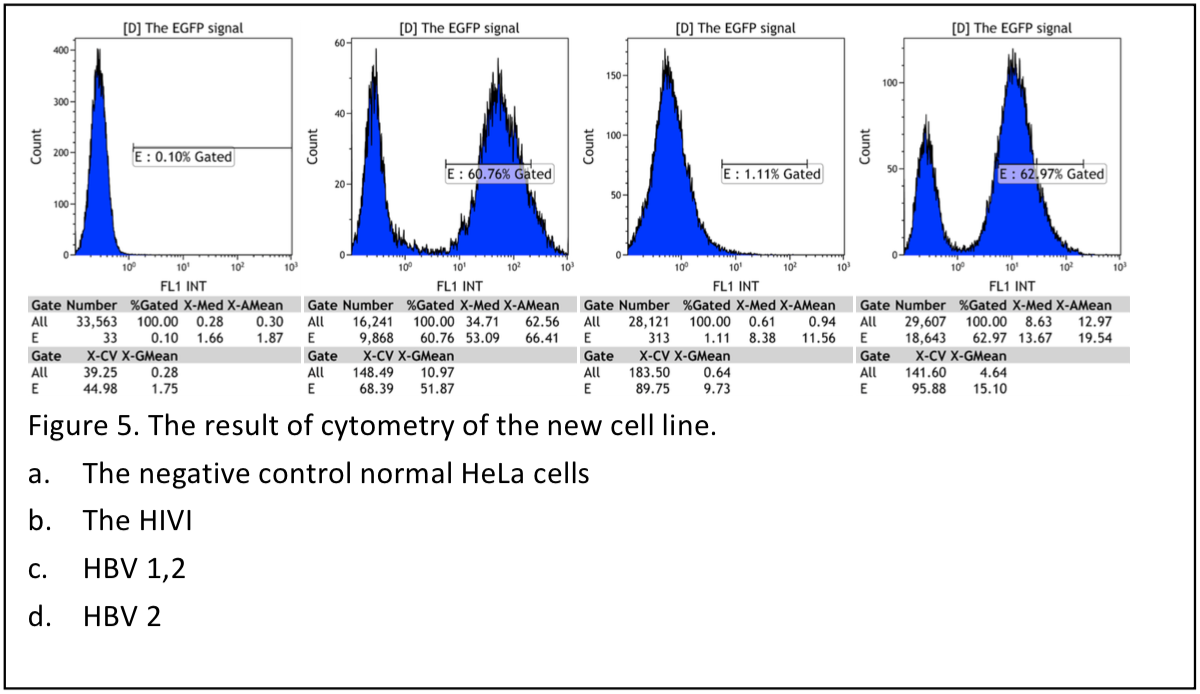
Result shows that the pool cells are not good enough to have further experiment, only 2/3 cells expressing green fluorecence.
 "
"